Oligonucleotide
synthesis reagents
Solvents and reagents
Deblocking / Detritylation
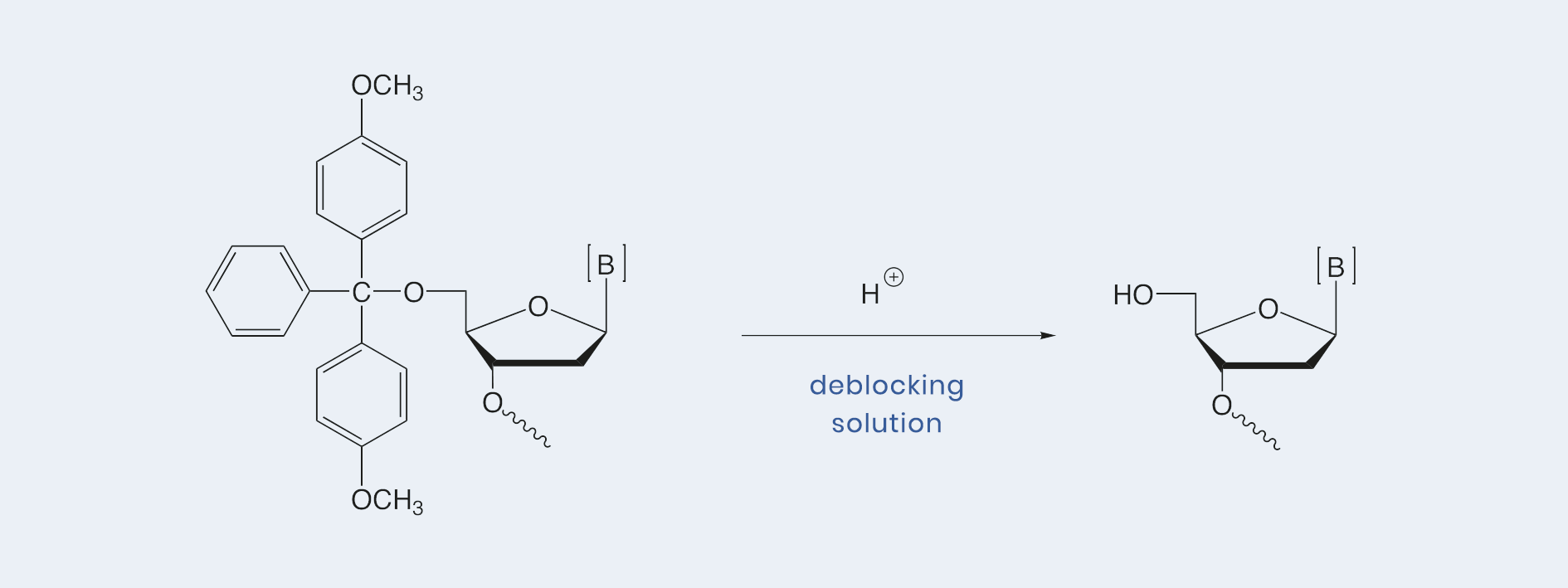 Deblocking solutions, used for the cleavage of the 5‘-DMTr group at the last building block of nucleotide chain, can consist either of dichloroacetic or trichloroacetic acid in dichloromethane or toluene. Our Hyacinth Deblocking solution (3 % dichloroacetic acid in toluene at < 30 ppm water) is applicable for the syntheses of high quality, large scale oligonucleotides.
Deblocking solutions, used for the cleavage of the 5‘-DMTr group at the last building block of nucleotide chain, can consist either of dichloroacetic or trichloroacetic acid in dichloromethane or toluene. Our Hyacinth Deblocking solution (3 % dichloroacetic acid in toluene at < 30 ppm water) is applicable for the syntheses of high quality, large scale oligonucleotides.
Activators
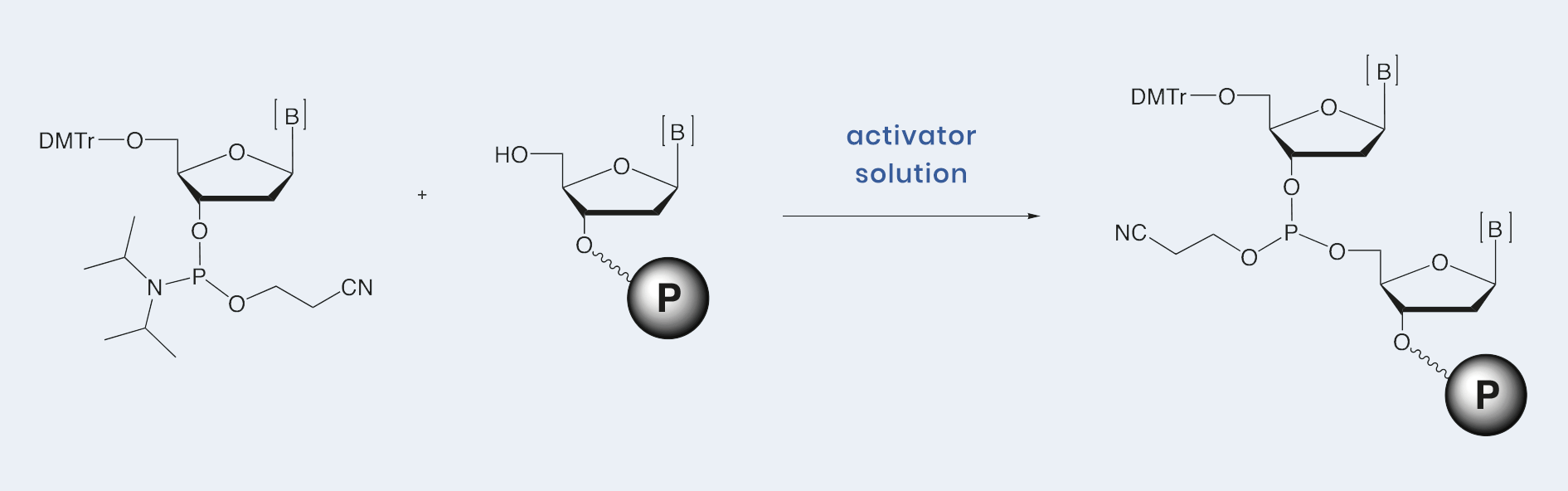 emp BIOTECH manufactures three different activators for use on various DNA and RNA synthesizers. They are available either in dry solid form for dissolution into anhydrous acetonitrile or as a prepared solution of various molarities.
emp BIOTECH manufactures three different activators for use on various DNA and RNA synthesizers. They are available either in dry solid form for dissolution into anhydrous acetonitrile or as a prepared solution of various molarities.
See all products
Hyacinth BMT
Hyacinth BMT activator (also known as 5-Benzylmercapto-1H-tetrazole or BTT)
demonstrates important advantages in the syntheses of oligonucleotides:
– Coupling efficiencies of 99 % with impeccable quality
– Lower percentage of n-1 sequences
– Dramatic reduction of coupling times to under 3 minutes in RNA syntheses
– Efficient RNA synthesis using 50 % or less TBDMS, TOM® or ACE® monomer
– Excellent batch to batch consistency for reproducible and stable oligo production
ETT
5-Ethylthiotetrazole (ETT) is an efficient activator for use in chemical synthesis of either DNA or RNA. ETT has excellent performance with respect to coupling times, coupling efficiency, consumption of phosphoramidites and reduction of n-1 impurities. ETT can be used for RNA synthesis with TBDMS, O-Methyl, TOM® or ACE® amidites.
DCI
4,5-Dicyanoimidazole (DCI) is an efficient activator
for use in chemical synthesis of DNA.
Capping Reagents
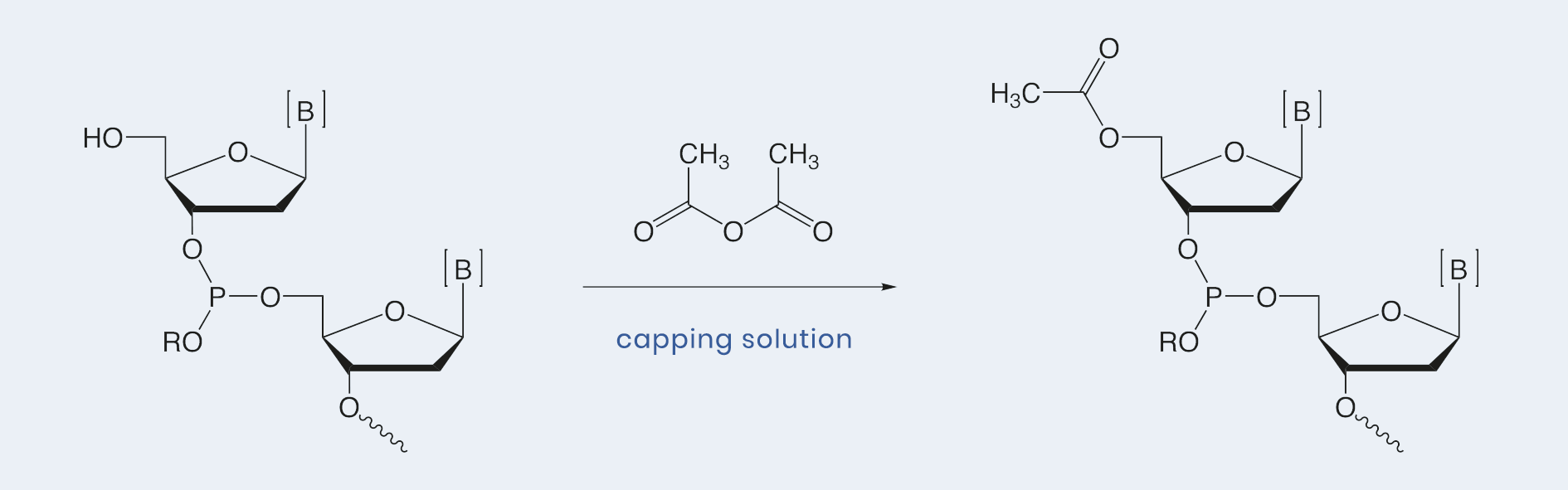 For each synthesis cycle, up to 1 to 2 % of free 5’-hydroxy groups remain after the phosphoramidite coupling step has been completed. By running a subsequent „Capping“ step using an anhydride, these free hydroxyl groups are converted to acetates and are hindered from further chain elongation and formation of long oligonucleotides with incorrect sequences.
For each synthesis cycle, up to 1 to 2 % of free 5’-hydroxy groups remain after the phosphoramidite coupling step has been completed. By running a subsequent „Capping“ step using an anhydride, these free hydroxyl groups are converted to acetates and are hindered from further chain elongation and formation of long oligonucleotides with incorrect sequences.
For optimal acetylation, a solution of acetic anhydride in Tetrahydrofuran or acetonitrile (Capping A) will be mixed in situ during reaction with a catalytic acting solution of N-methylimidazole (Capping B). Additives such as pyridine and lutidine function as mild bases to enhance the efficiency of the capping reaction.
See all products
Capping A
Capping B
Oxidizer
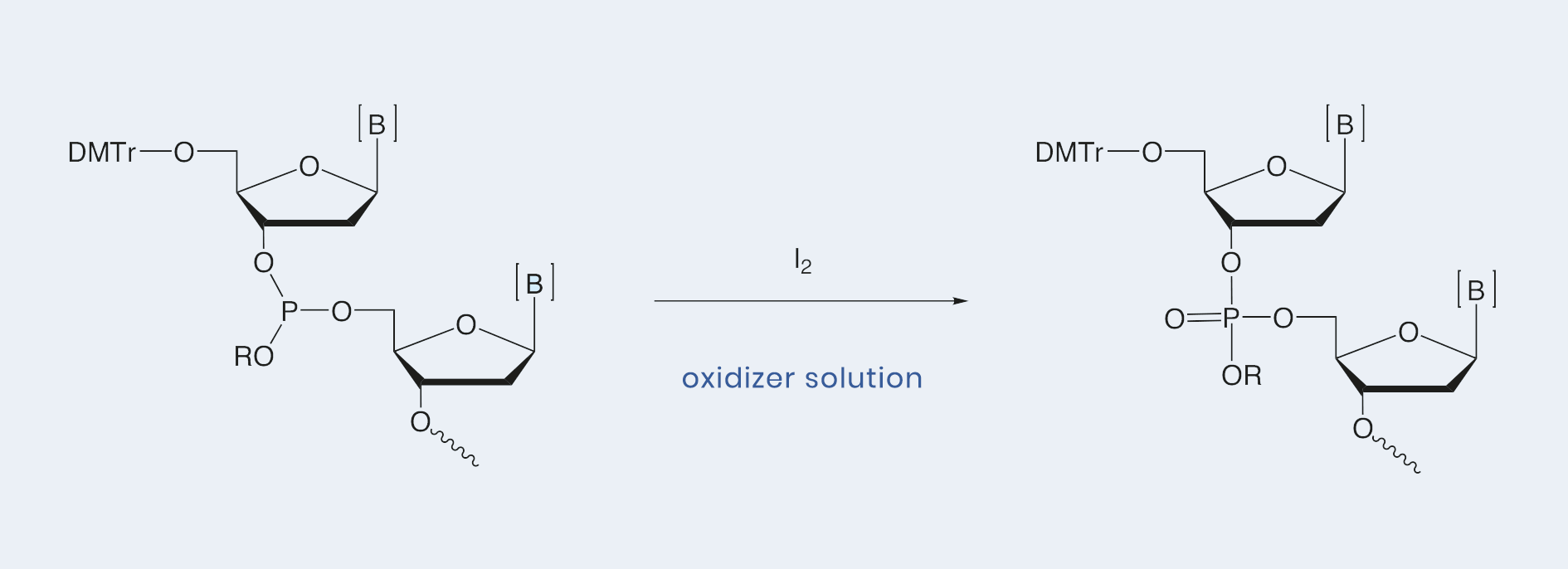 Oxidizer solutions promote the oxidation of trivalent phosphotriester into pentavalent phosphate triester using iodine as a mild oxidizing agent. They are available in standard 0.02 M and 0.05 M iodine concentrations with different mixtures of Tetrahydrofuran, pyridine and water. Custom mixtures are also available. The Hyacinth Oxidizer solution [0.05 M iodine in pyridine / water (V / V = 90 : 10)] is applicable for the syntheses of high quality, large-scale oligonucleotides.
Oxidizer solutions promote the oxidation of trivalent phosphotriester into pentavalent phosphate triester using iodine as a mild oxidizing agent. They are available in standard 0.02 M and 0.05 M iodine concentrations with different mixtures of Tetrahydrofuran, pyridine and water. Custom mixtures are also available. The Hyacinth Oxidizer solution [0.05 M iodine in pyridine / water (V / V = 90 : 10)] is applicable for the syntheses of high quality, large-scale oligonucleotides.
See all products
Cleavage and deprotection
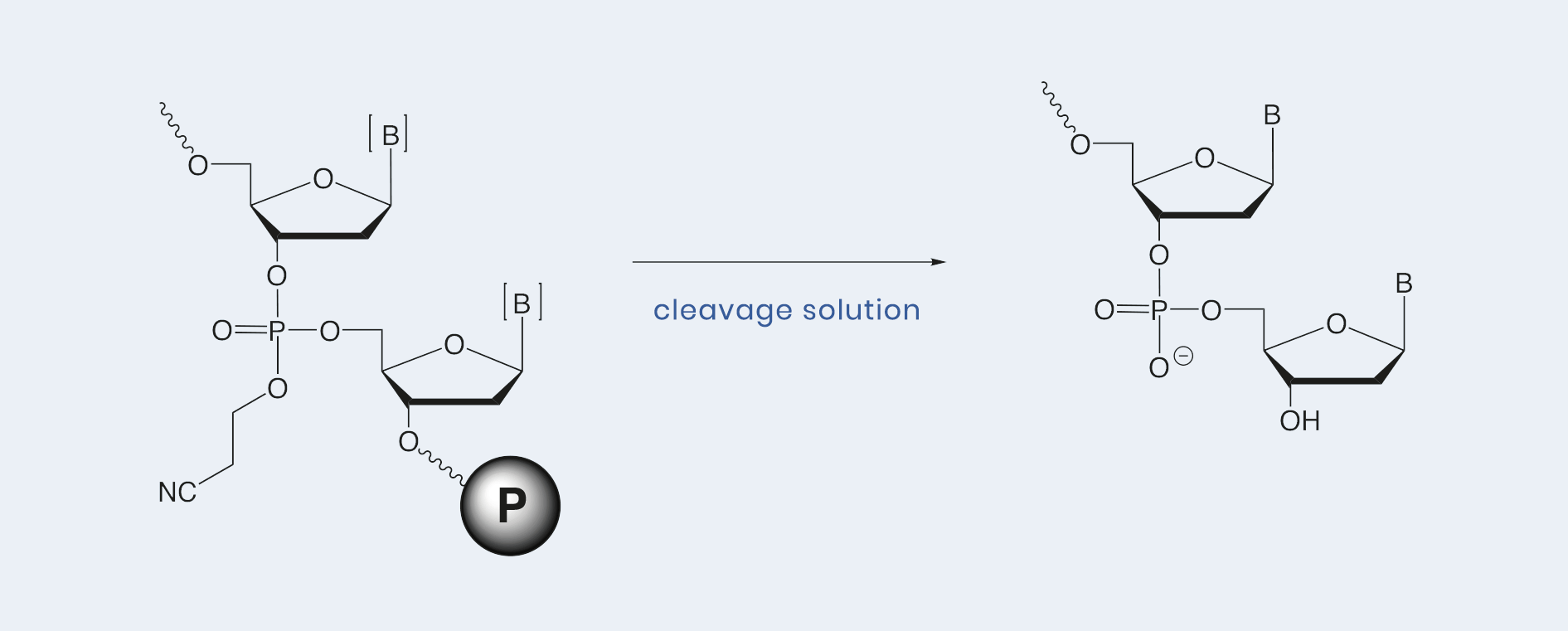 Cleavage of the oligonucleotide from its solid support and subsequent removal of all protecting groups from the nucleobases and phosphates close the cycle of automated oligonucleotide synthesis and bring it to completion. For this purpose, three different Cleavage solutions from emp BIOTECH are available. The correct choice will depend on your requirements for standard, fast or mild cleavage conditions.
Cleavage of the oligonucleotide from its solid support and subsequent removal of all protecting groups from the nucleobases and phosphates close the cycle of automated oligonucleotide synthesis and bring it to completion. For this purpose, three different Cleavage solutions from emp BIOTECH are available. The correct choice will depend on your requirements for standard, fast or mild cleavage conditions.
See all products
Standard
Usage of one volume of conc. ammonium hydroxide (= 28 %) under sealed conditions appropriate for removal of the protecting groups of the nucleobases
Fast
Usage of one volume of AMA under sealed conditions at 65 °C for 10 min
Mild
Usage of the required volume of Deprotection, Ultramild under sealed conditions for 8 h at room temperature
CE-ß-Elimination
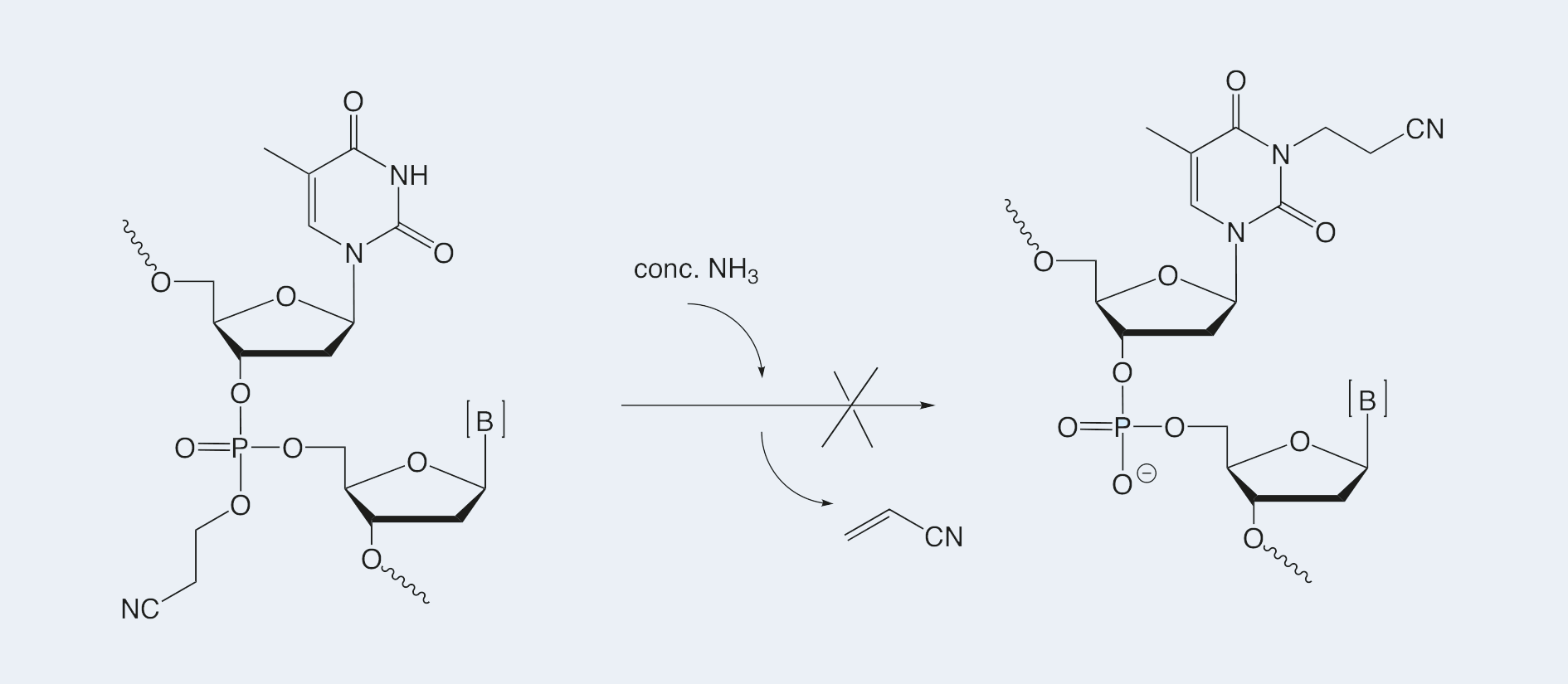 Alkylation of the N3-position of thymidine by acrylonitrile, which is liberated during ß-elimination of the cyanoethyl group from the phosphates, is a well-known side reaction during simultaneous cleavage of the protecting groups and the oligonucleotide from the support.
Alkylation of the N3-position of thymidine by acrylonitrile, which is liberated during ß-elimination of the cyanoethyl group from the phosphates, is a well-known side reaction during simultaneous cleavage of the protecting groups and the oligonucleotide from the support.
This side reaction can be avoided by use of diethylamine in acetonitrile for the ß-elimination. The oligonucleotide is subsequently cleaved from the support using any standard cleavage conditions.
See all products
Sulfurizing reagents
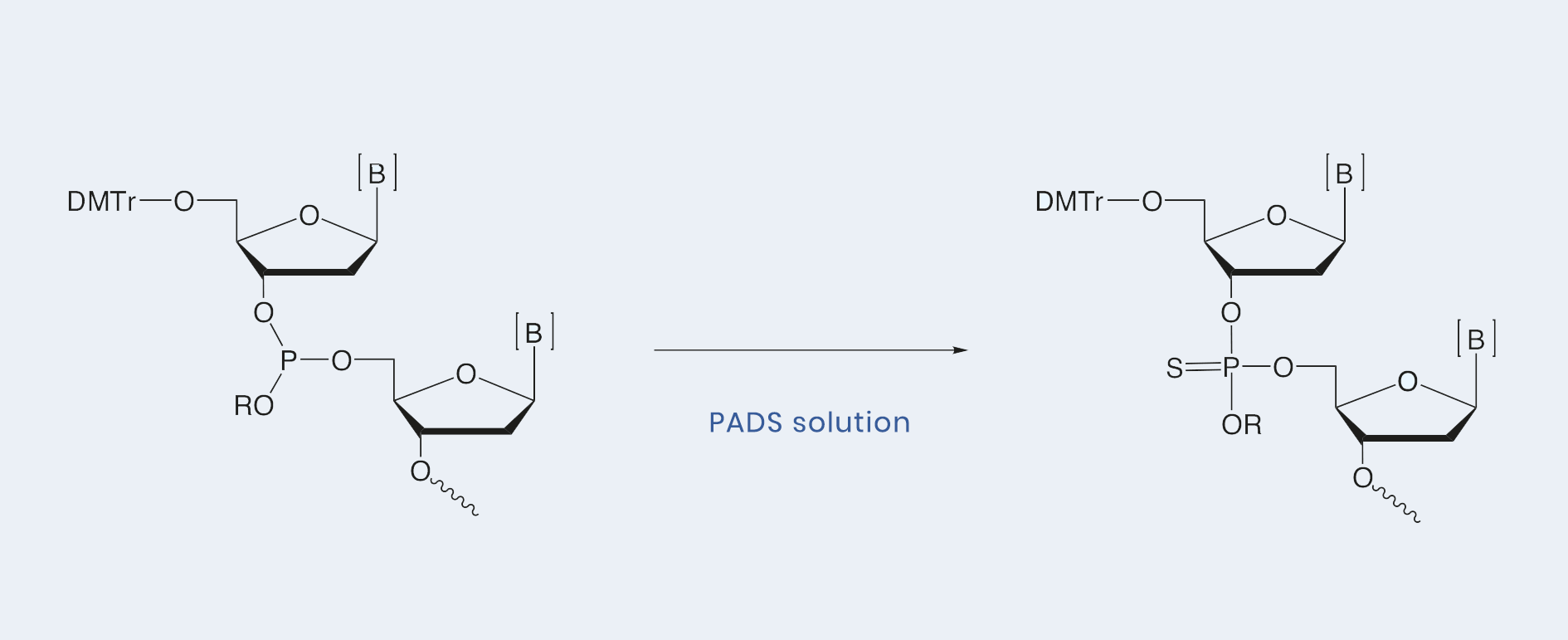 The phosphite triester formed in the coupling step can be converted to the corresponding phosphorothioate triester by treatment with 0.2 M solution of phenylacetyl disulfide (PADS) in acetonitrile and 3-picoline (V / V = 1 : 1). Typically, a 1.5-column volume of PADS solution is used, and sulfurization is complete within 3 minutes, at which time excess reagent is recovered from the reaction vessel by washing with acetonitrile.
The phosphite triester formed in the coupling step can be converted to the corresponding phosphorothioate triester by treatment with 0.2 M solution of phenylacetyl disulfide (PADS) in acetonitrile and 3-picoline (V / V = 1 : 1). Typically, a 1.5-column volume of PADS solution is used, and sulfurization is complete within 3 minutes, at which time excess reagent is recovered from the reaction vessel by washing with acetonitrile.
An alternative sulfur-transfer reagent is 3-amino-1,2,4-dithiazole-5-thione (ADTT, also known as Xanthane Hydride) which is suitable for solid-phase synthesis.
See all products
Solvents and solvent mixtures
| Product | Order No. | Unit Size |
|---|---|---|
| Acetonitrile (Water content = 20 ppm) | NC-0602-N002.5-001 | 2.5 L, GL45 thread |
| NC-0602-N004.0-G45 | 4 L, GL45 thread | |
| NC-0602-N004.0-U38 | 4 L, US38 thread | |
| Pyridine (Water content = 30 ppm) | NC-0604-N002.5-001 | 2.5 L, GL45 thread |
| Acetonitrile (Water content = 10 ppm) | NC-0609-M100.0-001 | 100 mL, 20 mm crimp/septum |
| NC-0609-N002.5-001 | 2.5 L, GL45 thread | |
| NC-0609-N004.0-G45 | 4 L, GL45 thread | |
| NC-0609-N004.0-U38 | 4 L, US38 thread | |
| Solvent Mix Pyridine in ACN (V / V = 60 : 40) | NC-0612-N002.5-001 | 2.5 L, GL45 thread |
Moisture control
Coming soon
Labeling and purification
Coming soon
Packaging specifications
Coming soon
